As Covid-battered India is eagerly awaiting the Pfizer vaccine, its neighbor Pakistan has already received the first batch of the American jabs.
The Pfizer vaccine is said to be “highly effective” for the dominant Indian variant of the Coronavirus. The country is still in the grip of a devastating second wave of the Covid-19 pandemic.
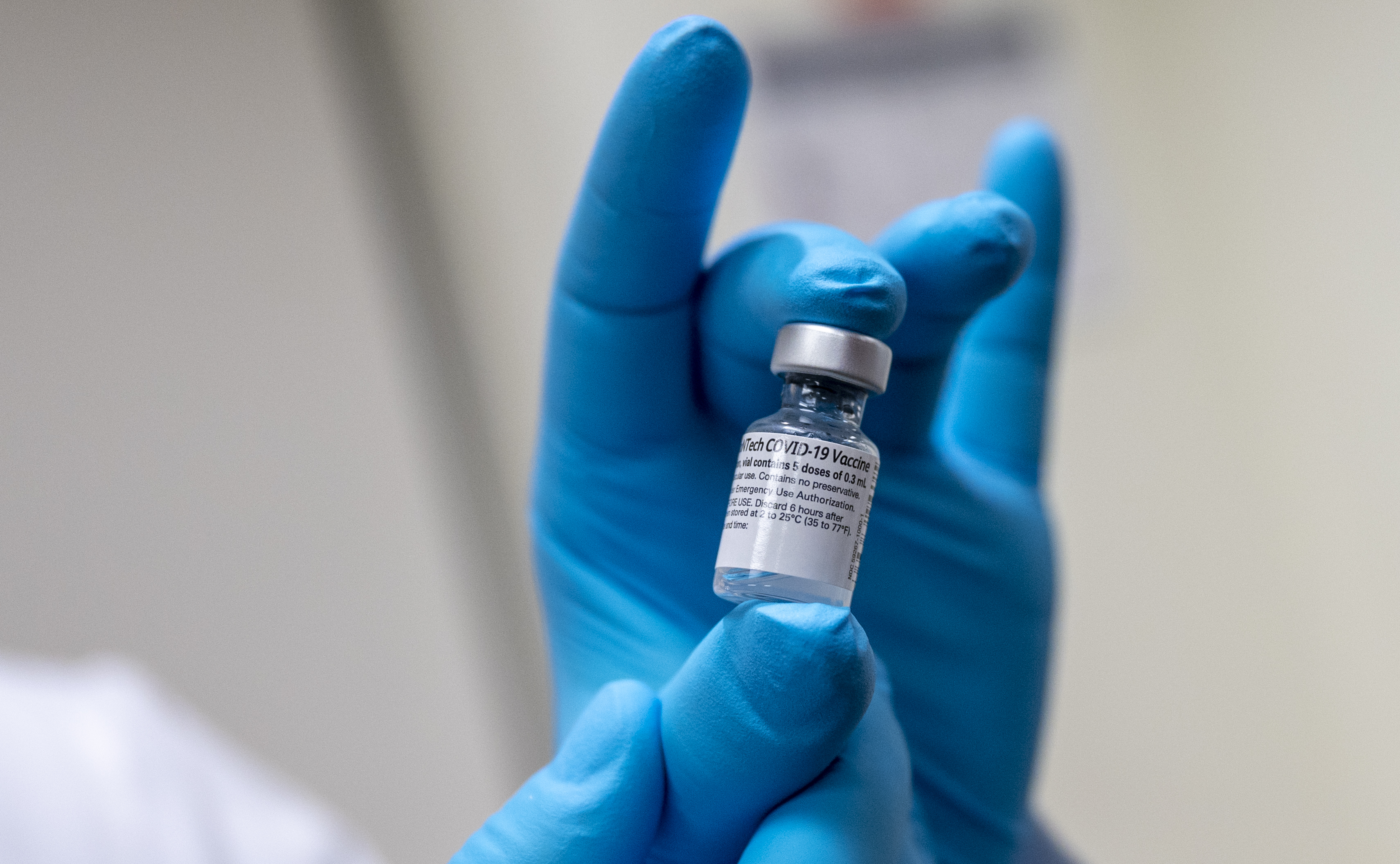
According to UNICEF, Pakistan has already received 100,000 doses of Pfizer vaccine through the World Health Organization’s Covax initiative. This is the second shipment of vaccines Pakistan has procured through Covax, Dawn reported.
The first shipment comprising over 1.2 million doses of AstraZeneca-Oxford vaccines had reached Pakistan on May 8.
Established in April 2020, Covax is an initiative launched by Global Alliance for Vaccines and Immunisation (GAVI), Coalition for Epidemic Preparedness Innovations, and WHO. The alliance has promised the supply of free vaccines to at least 20 percent of the population in countries around the world.
Pakistan is expected to receive a total of 17.2 million doses through Covax.
UNICEF Pakistan has lauded Pakistan’s successful launch of the Covid-19 vaccination drive. Starting May 28, the registration has been opened for people over 18 years having the Computerized National Identity Cards (CNICs).
Over 100,000 doses of @pfizer #COVID19 vaccine via #COVAX have arrived in Pakistan ??. Shipments of @pfizer diluents and syringes will be arriving over the next two days. The vaccines will be used for @GovtofPakistan's ongoing #COVID19 vaccination campaign.@nhsrcofficial pic.twitter.com/Ps4Qmnk1np
— UNICEF Pakistan (@UNICEF_Pakistan) May 28, 2021
Pakistan has approved five Covid vaccines so far — Oxford’s AstraZeneca, the Russian Sputnik and China’s Sinopharm, Cansino, and Sinovac.
What’s Causing Delay In India
India’s apex policy think tank, the NITI Aayog, has stated that the federal government is in talks for acquiring the Pfizer Covid-19 vaccine. It is expected that India may receive the vaccine by July 2021.
The US vaccine maker had reportedly sought indemnity against the cost of compensation for any severe side effects.
So far, India has not granted indemnity to any vaccine maker. The country has so far approved three vaccines — Covishield, Covaxin and the Russian Sputnik.
“We are looking at what Pfizer’s expectations from the government are, and they are looking at what our expectations from them are. That is the process in which it will move because they have to come into India; they have to apply for licensure,” Dr. VK Paul, who heads the government’s Covid vaccine task force, was quoted as saying by The Indian Express.
The American company has found its mRNA vaccine “highly effective” against the dominant coronavirus variant in India. It has also been proven suitable for children over 12 and may prove helpful in expanding the vaccination drive to those below 18.
On May 28, the European Union approved the Pfizer/BioNTech coronavirus jabs for 12 to 15-year-olds, the first vaccine to get the green light for children in the bloc, AFP reported.
Besides, the vaccine can be stored in a cold storage facility at temperatures 2-8 degrees Celsius for a month. On its part, the Indian government has indicated that it is looking at the cold chain and supply requirements of the vaccine.
The government maintains that it is making continuous efforts to procure Covid-19 vaccines from foreign manufacturers. It is also trying to increase the supplies of the Sputnik vaccine.
The Sputnik V is the third vaccine to be approved in India and is expected to be available through the Apollo Hospitals Group from the second week of June.
To increase the vaccination drive in the country, the government is also trying to increase the production of vaccines currently being manufactured in India.
According to government estimates, Bharat Biotech is expected to increase its production capacity of Covaxin by almost 10 times. The Hyderabad-based company had started with a production of 90 lakh doses per month and may increase the production to about 10 crore per month in the coming weeks.
A similar increase is also expected in the production of the Covishield vaccine. The Serum Institute of India aims to increase the manufacturing of its Covishield vaccine to almost 11 crore doses by September and October 2021. It is currently manufacturing 6.5 crore doses a month. The government maintains that in July the shortage of vaccines in the country will be over, and almost 51.6 crore doses of vaccines will be available.




